

The TricValve system gained breakthrough device designation by the FDA in December 2020.
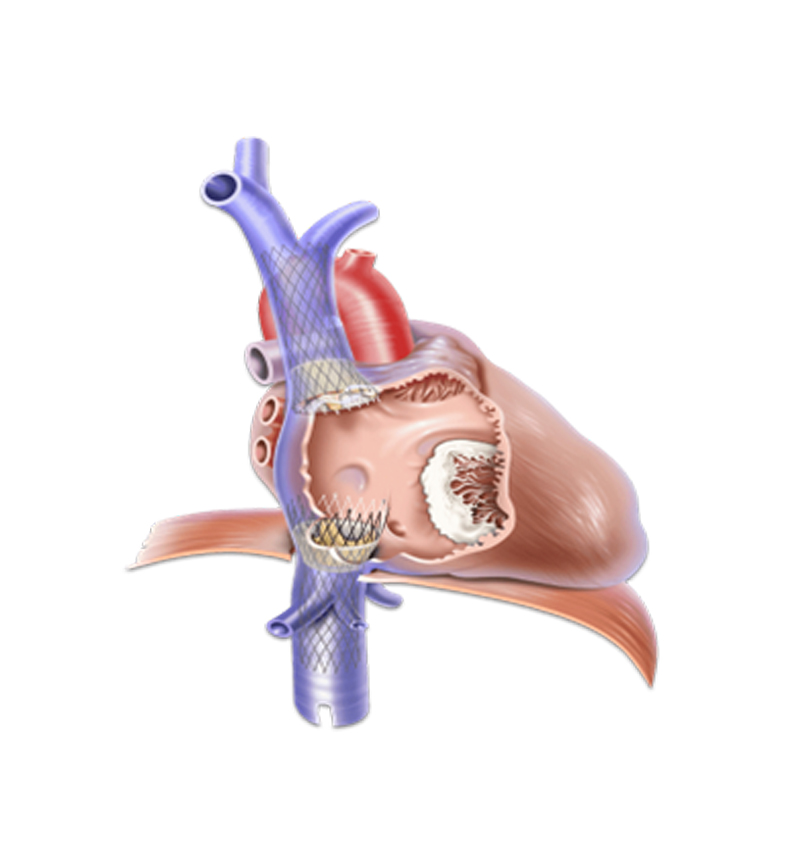
It consists of two self-expanding biological valves that are implanted percutaneously into the superior vena cava (SVC) and inferior vena cava (IVC) without disturbing the native tricuspid valve.
The caval system is intended for patients with severe symptomatic Tricuspid regurgitation and Right heart failure who are deemed to be at extreme/high surgical risk or noncandidates for open surgical therapy.
The TricValve system is designed for patients with severe symptomatic TR (tricuspid regurgitation) and RHF (right heart failure) who are considered to be at extreme/high surgical risk or are not suitable candidates for open surgical therapy. It offers a less invasive alternative for such patients.
The key features of the TricValve system include:
1. Two Self-Expanding Biological Valves: The system consists of two biological valves that are designed to be self-expanding. These valves are intended to be implanted percutaneously, which means they are inserted through the skin or a body opening, into the superior vena cava (SVC) and inferior vena cava (IVC). This approach aims to address tricuspid regurgitation without disturbing the patient's native tricuspid valve.
2. Targeted Cavas: The valves are specifically implanted in the superior vena cava (SVC) and inferior vena cava (IVC), which are major veins that carry deoxygenated blood from the upper and lower body back to the right atrium of the heart, respectively. This positioning suggests that the device is designed to help regulate blood flow and reduce regurgitation in the tricuspid valve.
The breakthrough device designation by the FDA highlights its potential as a promising advancement in the treatment of these cardiac conditions.